Engineers who Deliver Manufacturing Excellence
Since 2006, our team of seasoned experts has been transforming the FMCG and pharmaceutical industries with a powerful blend of technical advisory and hands-on project implementation.
We don’t just offer solutions – we drive success, ensuring every project thrives from strategy to execution, guided by our core values of simplicity clarity and integrity.

About Us
Since 2006, our team of seasoned experts has been transforming the FMCG and pharmaceutical industries with a powerful blend of technical advisory and hands-on project implementation. We don’t just offer solutions – we drive success, ensuring every project thrives from strategy to execution, guided by our core values of simplicity clarity and integrity.
We are a dynamic technology and engineering consultancy powered by a team of 40+ experts and 20 seasoned associates. Our engineers, consultants, and project managers bring a unique blend of hands-on industry experience and strategic consulting expertise – delivering innovative solutions that drive real impact.
JPH works with clients of all sizes across food & drink, personal care, and pharmaceuticals – partnering with manufacturers, brand owners, packaging suppliers, distributors, financial institutions, and private investors to drive growth and success.
Meet our leadership teamWhy Partner With Us?
Turning business & technical challenges into growth opportunities.
Driving innovation & operational excellence for industry-leading blue-chip clients.
Applying proven methodologies & client-focused solutions to optimise outcomes.
Guidance by chartered engineers, consultants and certified project managers combines real-world experience with strategic insight to deliver lasting impact.
From strategic advisory to on-site execution and implementation – your trusted partner for sustainable growth & success.
We understand the complexities of manufacturing and know how to turn challenges into results.

Employee Owned
Our company is proudly employee-owned, ensuring that our team’s success is directly aligned with our clients’ best interests. As an independent firm – free from supplier, contractor, or external consulting ties – we deliver truly impartial, unbiased expertise on every project.
As an employee-owned business, our people are truly invested in your success. With a shared sense of ownership, they go above and beyond to deliver exceptional service – driven by pride, passion, and a commitment to exceeding client expectations.
In November 2024, we were proud to be named ‘EO Rising Star of the Year’ at the prestigious UK Employee Ownership Awards – a powerful recognition of our people-first culture and unwavering dedication to delivering outstanding results for our clients.
Our Services
Explore our comprehensive suite of services designed to create real value for our clients:

Technology & Innovation
We deliver breakthrough innovations in product technologies, manufacturing processes and packaging to drive market leadership.
We drive innovation and commercialisation by scouting emerging technologies, validating concepts through pilot testing and scale-up, and assessing feasibility and risk.
Our services include strategic roadmapping, IP strategy, benchmarking, and open innovation — enhanced by AI tools and knowledge management to accelerate outcomes and reduce development risk.
Core Capabilities
- Innovation strategy, technology scouting & roadmap development
- Feasibility assessment, PoC testing & risk analysis
- IP strategy, commercialisation & technology transfer
- AI-driven decision support, knowledge & data management
- Open innovation, partnerships & competitive benchmarking

Engineering & Implementation
We provide end-to-end engineering solutions, from custom process design to seamless implementation, optimising FMCG manufacturing performance and efficiency.
We deliver integrated engineering solutions from concept through to turnkey implementation, combining regulatory expertise, sustainability, and automation to optimise manufacturing performance and ensure compliance.
Our services include process design, design validation, system installation and commissioning, alongside technology transfer, regulatory support, and digital integration—enabling reliable, efficient, and future-ready operations.
Core Capabilities
- Process design, validation, and turnkey implementation
- Regulatory compliance and risk management (FDA, 9001, HACCP)
- Technology transfer, scale-up, and specialised equipment integration
- Advanced automation, digital solutions, and data connectivity
- Sustainable, energy-efficient design with focus on safety, ergonomics, and lifecycle performance

Automation & Digital Solutions
We optimise product manufacturing with automation, digital tools, and IT solutions, enhancing efficiency, compliance, and real-time data management.
We help manufacturers unlock digital transformation by integrating intelligent automation and control technologies across operations. Our approach supports system modernisation, real-time visibility, and improved decision-making through IT/OT convergence and the deployment of smart sensors, IoT, and digital twins.
Our services include implementation of Manufacturing Execution Systems (MES), traceability solutions, AI-driven process optimisation, and cloud-based architectures for scalability. We also deliver advanced robotics and autonomous systems to enhance productivity, reduce downtime, and future-proof manufacturing environments.
Core Capabilities
- Automation, control systems & smart sensor integration
- Digital manufacturing, IT/OT convergence & cloud infrastructure
- MES, traceability & real-time monitoring solutions
- AI, machine learning & digital twin technologies for optimisation
- Robotics, autonomous systems & system modernisation

Strategic Business Advisory
We provide expert business planning and strategic advisory services to help FMCG companies make informed decisions and drive long-term success.
We provide strategic advisory and planning support to help FMCG businesses navigate growth, innovation, and transformation. Our approach enables informed decision-making, stronger market positioning, and long-term success.
Our services include market and consumer analysis, financial forecasting, business case development, risk management, regulatory and sustainability guidance, technical due diligence, process scale-up support, digital transformation, innovation strategy, and M&A advisory.
Core Capabilities
- Market analysis, & consumer insights
- Financial modelling, business case development & M&A advisory strategies
- Risk assessment, technical due diligence & compliance strategy
- Process scale-up, sustainability & regulatory readiness
- Digital transformation, innovation & growth planning

Process & Performance Optimisation
We deliver performance improvements, troubleshoot operational challenges, optimise processes to enhance efficiency and boost productivity in FMCG operations.
We drive robust performance improvement by combining data-driven methods with scientific and engineering expertise. Using tools like root cause analysis, process mapping, lean manufacturing, and Six Sigma, we systematically identify and address inefficiencies to enhance productivity and reliability.
Our services include automation and digital tool implementation, process simulation, predictive maintenance, and advanced analytics—enabling real-time decision-making, improved operational precision, and continuous optimisation.
Core Capabilities
- Lean manufacturing, Six Sigma & statistical process control
- Root cause analysis & process mapping
- Data-driven decision making & predictive maintenance
- Automation, digital tools & process simulation
- Integrated scientific and engineering problem-solving approach

Quality & Compliance Management
We develop and implement solutions to ensure product quality, safety, and compliance, helping FMCG businesses maintain consistency, mitigate risks, and meet industry standards
These capabilities focus on implementing robust Quality Management Systems (QMS), managing risk, and standardizing process control to ensure consistency and compliance. With advanced testing, validation, and digital quality monitoring, we enable accurate, data-driven quality assurance across manufacturing operations.
Our services include smart quality control systems, equipment and process validation, sanitation verification, incident investigation, and cultivating a strong quality culture through leadership engagement—supporting regulatory compliance, continuous improvement, and high operational performance.
Core Capabilities
- Quality management systems, process control & standardisation
- Risk assessment, incident investigation & root cause analysis
- Product, equipment & sanitation validation
- Digital quality monitoring & smart automation systems
- Quality culture development & leadership engagement

Project & Vendor Management
We ensure efficient, compliant, and cost-effective projects through expert management and vendor coordination
These capabilities reflect a comprehensive approach to delivering complex projects by integrating end-to-end program management with effective stakeholder engagement, vendor oversight, and regulatory compliance. With strong budget control, risk mitigation, and performance monitoring, organisations can achieve efficient, compliant, and cost-optimised project delivery.
Our services include construction governance, technology transfer, change management, and supply chain coordination—ensuring smooth execution, continuous improvement, and alignment across all project phases.
Core Capabilities
- Full-scope project and program management, including construction and facilities governance
- Stakeholder engagement, vendor management & governance
- Regulatory compliance, risk mitigation & performance monitoring
- Budget control, cost optimisation & continuous improvement
- Technology transfer, change management & supply chain coordination

Infrastructure & Construction Oversight
We manage the design, construction, and compliance of production facilities, ensuring regulatory compliance, optimised utilities, and strict health & safety standards
These capabilities encompass full support for facility development, covering expert design and engineering, construction project management, and integration of utilities and process systems. They ensure compliance with cGMP, FDA, EMA, ISO 14644, and CDM 2015 standards while embedding risk management, emergency planning, and business continuity strategies.
Our services include health, safety, and environmental (HSE) compliance, as well as sustainable, energy-efficient facility solutions tailored to deliver long-term operational excellence.
Core Capabilities
- Facility design, engineering, and construction project management
- Regulatory compliance and cGMP facility qualification (FDA, EMA, ISO, CDM)Risk management, emergency planning, and business continuity
- Utilities, process systems integration, and HSE compliance
- Sustainable and energy-efficient facility solutions
Our Services
Explore our comprehensive suite of services designed to create real value for our clients:
Manufacturing Excellence
We take responsibility for delivering major capital engineering and manufacturing improvement projects.
Capacity
Optimising production planning; new production lines for new products; and removing constraints, are typical capacity expansion projects that we deliver.
Cost
Rationalising production capacity; automation; increasing yield and reducing waste through intelligent design and planning, are typical examples of cost-saving projects that we deliver.
Quality
Working in food and pharma means that all our projects have the highest quality standards. We qualify and validate all our own projects.
Compliance
Our knowledge of pharma and food regulations together with health and safety means that we are often asked to assess, recommend and deliver compliance projects.
Support
Factory infrastructure replacement, and streamlining support services, are projects that we deliver to mitigate risk and improve business efficiency to move closer to our goal of manufacturing excellence.
Operational Excellence
Smart Factory is on our doorstep and it’s not going away anytime soon. It provides the opportunity to use modern technology to make data centred decisions. Here at JPH we have a plethora of tools at our disposal to assist you in the Smart Factory journey.
MXL
MXL Smart Factory software is a flexible tool which enables you to model your factory from end to end, create live or static digital twins, scenario plan and more. Using MXL, we can make comparative costs and benefits across equipment, labour and energy, therefore facilitating investment decisions.
Data Collection
Rationalising production capacity; automation; increasing yield and reducing waste through intelligent design and planning, are typical examples of cost-saving projects that we deliver.
Data Mapping
A key step in data management. We work with you to ensure that data flows correctly throughout your organisation by looking at Data Architecture and Database Design.
Smart Factory Assessment
What is your Smart Factory IQ? We use our expertise to assess your current status as a Smart Factory. By performing technical reviews and gap analyses, we start to build a picture of the scope of works.
Workshop
We speak to your employees, ranging from line workers to senior management, about their daily challenges and what their vision of a Smart Factory would encompass. We also relay the message that the Smart Factory is a tool not a threat.
Road Map Development
Presenting our findings and working with you to build a clear timeline for the Smart Factory journey – including a Cost/Benefit analysis.
Pharmaceutical Excellence
We provide one-stop, expert support to the pharma, biopharma and medical devices industries, delivering both turnkey and standard solutions.
Target-Driven Delivery Ethic
We offer a professional and well-practiced service, resulting in complete solutions that fulfil the specific requirements of all our clients, whatever the sector, whatever the challenge.
One Stop, Full Product Lifecycle Coverage
Our portfolio of services covers all stages of the product lifecycle and is delivered by a team of specialists including chartered engineers, quality professionals, and accredited project managers with extensive sector knowledge and pharma experience.
Quality Driven
We apply the principles of QMS by applying validation, qualification, compliance and GMP. We combine this with collaboration and knowledge sharing to appreciably improve overall quality resulting in more efficient safe regulated improved products and processes.
Covering Start-up to Fully Established Enterprises
We apply the principles of QMS by applying validation, qualification, compliance and GMP. We combine this with collaboration and knowledge sharing to appreciably improve overall quality resulting in more efficient safe regulated improved products and processes.
Culture
We pride ourselves in forming long-lasting relationships as well as the results we achieve. We welcome the opportunity to explore building new partnerships with organisations such as yours.
We love solving challenging technical problems in complex manufacturing environments. From fast moving consumer goods, to high tech pharmaceutical production our team relish the challenge of identifying and delivering major investments in innovative companies.

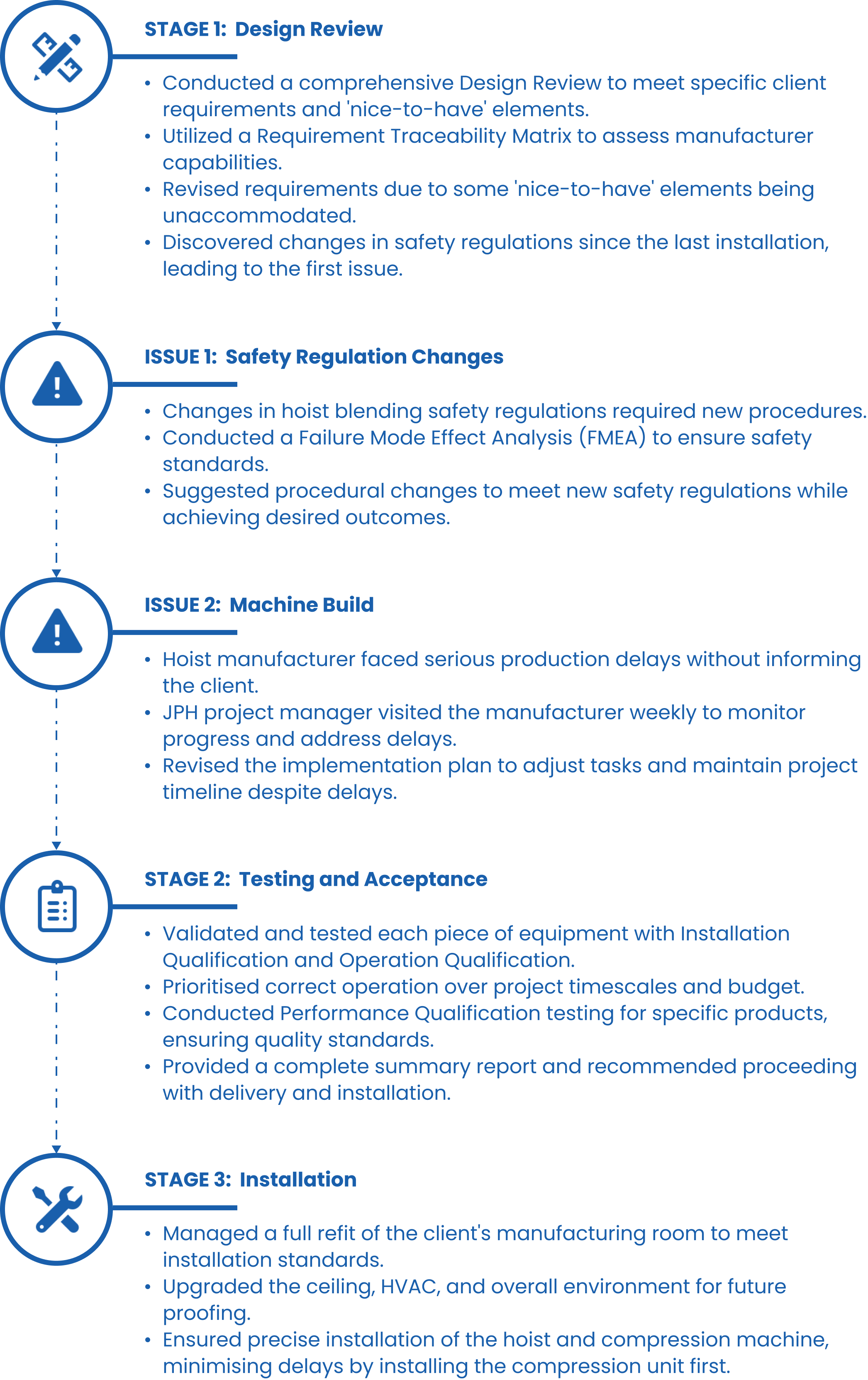
Deep Dive: The Pharmaceutical Expansion Plan and Re-Plan
Our pharmaceutical client needed to double tablet production – but the disruption caused by manufacturer delays would need some expert support…
JPH’s work on this particular project began in 2016. Our client – a UK-based pharmaceutical manufacturer – needed to double their output of tablets and planned to do this by duplicating the equipment and procedures they already had in place. The main features of this equipment were a drum blender and hoist, and a tablet compression machine – with all of the installation, compliance and safety standards that those intricately-calibrated devices require.
This type of installation requires detailed analysis, quality assurance and tight project management: every key part must be manufactured, tested and installed to the highest standards, as there is no room for error. Lacking the resources to dedicate to this project themselves, they approached us to take on delivery, and all that would entail.
Despite the challenges that the supplier delays presented, we were able to double tablet production to all of the vital industry and safety standards required. With monthly stakeholder meetings, and weekly project team meetings with the client as well, we were able to give them the reassurance and confidence they needed that the project would be delivered – with all the due diligence and close supplier management that we brought to the project as well.
That successful production has continued since this project concluded in 2017 – and our relationship with that client has continued as well, as we deliver larger and even more complex production line projects, and act on their behalf as liaison with machine manufacturers.
Deep Dive: From Covid Crisis to Long-Term Partnership
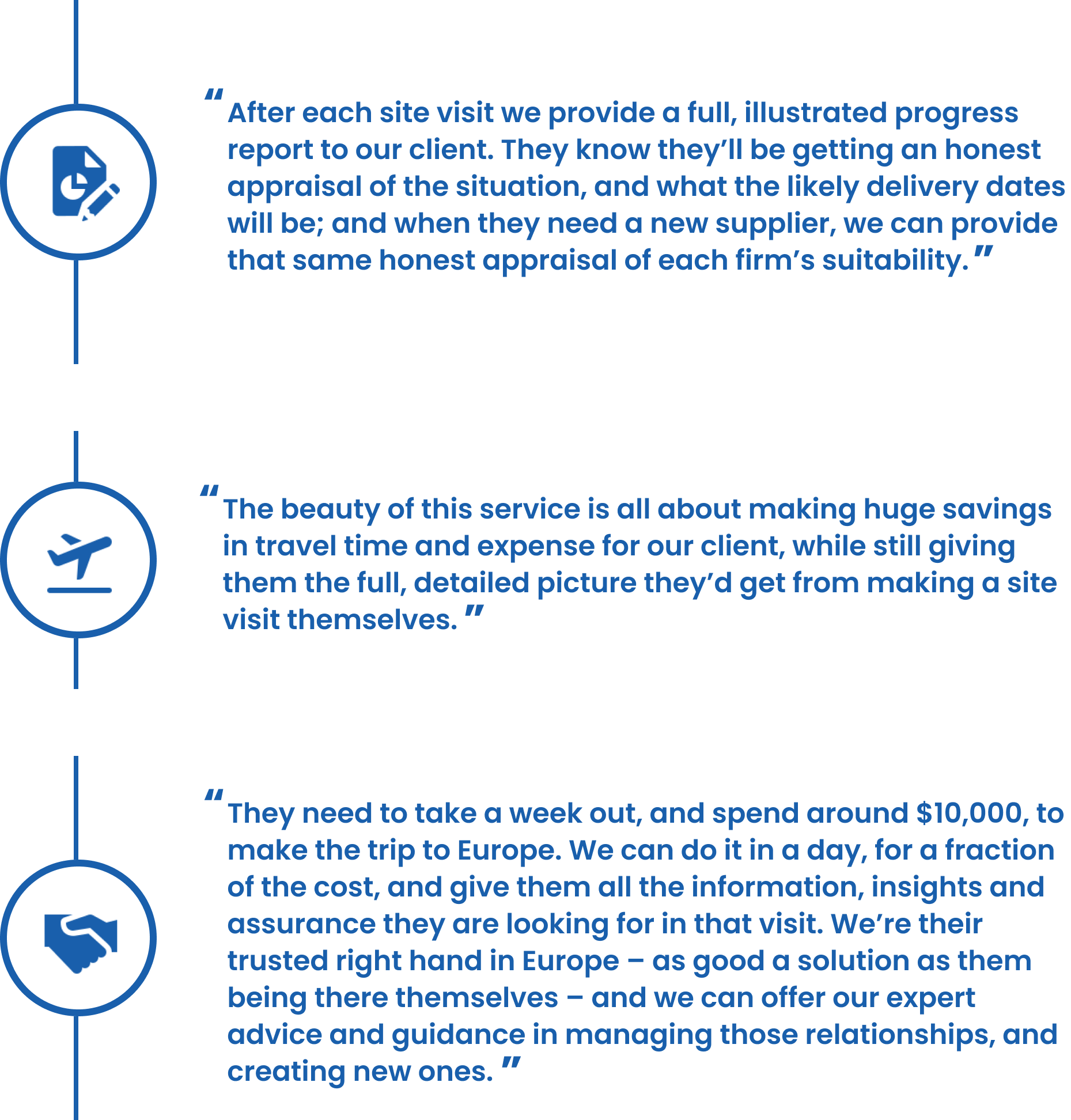
On the ground when our clients couldn’t be: when Covid19 restricted all travel from Australia, we stepped in to be their eyes, ears and voice with suppliers…
This JPH client is a major manufacturer of biscuits and confectionery in Australia. They source their production machinery from suppliers in Europe – and when Covid19 locked down the Australian borders for many months, this became a serious issue for them. Prepandemic, our client would travel to be on the ground at their supplier sites in Europe, personally overseeing build quality, testing and sign off on the finished product ready to ship back home to Australia. That presence is vital for our clients to be satisfied of the standard and quality of the build, for the size of the investment they’re making.
So when travel became out of the question in 2020, this posed a significant risk. They could no longer be sure that the machines they’d ordered were being built to those time, spec and quality requirements, because they simply could not be there to check. They needed someone in Europe who could act as their trusted proxy: specialists who were nearby, who could travel within the restrictions, with the same level of knowledge and understanding about the necessary quality standards.
That’s when they reached out to JP Hildreth, and we assigned them their own case manager: a single point of contact who could co-ordinate visits to their European suppliers in a day, check on quality, and report back to them comprehensively on progress – as good as them being there themselves. And although this was initially to be a Covid-related solution, we’ve continued to work on our client’s behalf here in Europe, even since the lifting of those travel restrictions: we are their experts on hand in the northern hemisphere.
Deep Dive: The Packing Production Puzzle
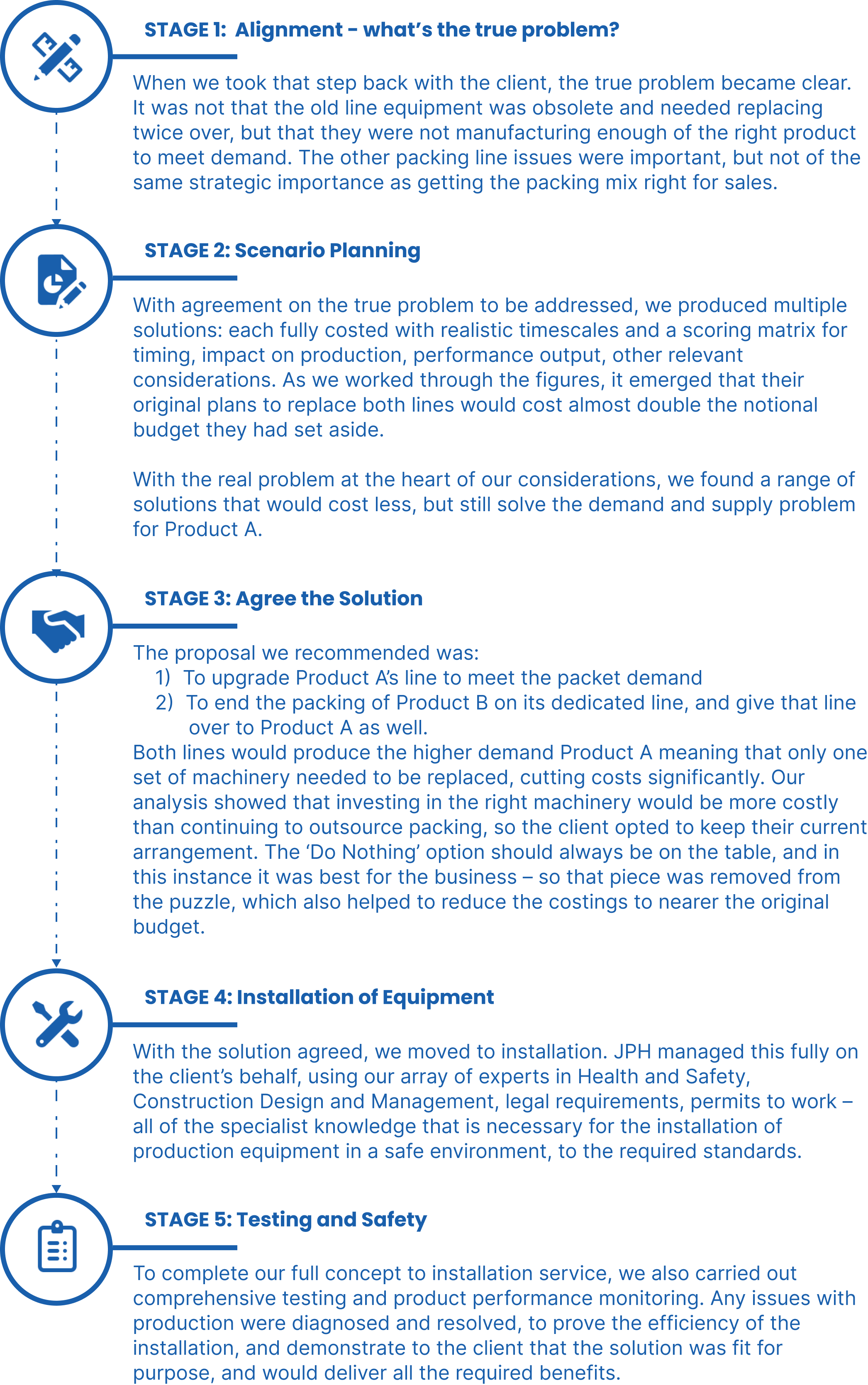
When demand outstrips supply, there are several paths to take. This is how we solved the Packing Production Line Puzzle…
Our client is a major manufacturer, and a new customer preference in packaging was becoming a trend they couldn’t ignore. Sales of their product sold in packets, rather than traditional boxes, were far outstripping the others; and they needed to boost the numbers of packet products to meet that demand. Our client knew they would need to make changes within their production lines to address this, along with some other issues to solve. Here’s a breakdown in summary of the puzzle in front of them:
- Product A: High demand for packets over boxes, with obsolete box machinery. Unsuitable cases requiring unnecessary repackaging.
- Product B: Lower sales than product A and distributed only in the UK produced with outdated packet machinery in need of replacement.
- Increasing packet demand with no way to change the existing production line pack mix to meet that demand.
- No room to install any new equipment alongside the current lines.
These were the problems to solve. With no room for extra packing lines, the client planned to replace Product A’s line with new machinery that would both solve the case problem, and increase packet production capabilities. They also proposed a replacement to Product B’s line machinery at the same time – so two full-scale line replacements, with a notional budget of £5million to achieve them.
From the earliest stage of knowing a change was needed, JPH were able to offer objective, expert support for decision-making, followed by a fully managed installation service. Our clients could have 100% confidence that their major investment would return the greatest benefit to them, and that their new equipment was installed to all industry standards – without needing to have any of that expertise in-house.
Our Clients
We are privileged to work with the most forward-thinking global manufacturing companies.
Our reputation for putting clients first and always looking for opportunities to improve manufacturing performance means that most of our work is repeat business from people who trust us to do the right thing.
Contact us
[gravityform id=1 title=false description=false ajax=”true”]